We succesfully had a virtual event of Animal Health Innovation Asia 2020 by Kisaco Research. November 25th was Japan Day, and thanks to Mr. Calan Smith, I was honored to host 3 programs, i.e. “Panel Discussion” by Messrs. Kato, Fukui and Hagihara who are industry leaders from Zoetis Japan, Zenoaq, and Kyoritsu Seiyaku, respectively, “Innovation Showcase” by 4 start-ups, such as Toletta Cats, MT3, Vetamic (Nihon Univ.), and KAICO, and “University Pavilion” by 3 universities, such as Prof. Mizuno of Yamaguchi Univ., Assoc. Prof. Isaka of Rakuno Gakuen Univ., and Dr. Kato of Tokyo Univ. of Marine Science and Technology.
We hope that we can have an offline event, not a virtual one, in Tokyo in 2021.
Introduction of AHRMS, Inc.
In advance for “Animal Health Innovation Asia 2020” by Kisaco Research, we prepared an introductory video 🙂
I hope it can be of help to your understanding of our company’s services.
- The program “Animal Health in Japan” on November 25th during “Animal Health Innovation Asia 2020”
- Corporate brochure:


- Regulatory Pathways and Market Access:

Animal Health Innovation 2020
Thanks to Kisaco Research, the two pre-events of Animal Health Innovation Asia 2020 were successfully conducted on November 3rd and 10th. According to Mr. Stephen Swarray of Kisaco, 300 people joined the pre-event “Next Steps for Animal Health in Asia” by Zoetis. Of course, I really enjoyed both, too.
“How to consult with veterinary clinics and new-style cat lovers’ lives that suit the “With COVID-19” years.
At the request of Toletta Cats, Inc. that develops and markets a wonderful IoT cat litter “Toletta”, I wrote the above-captioned column for cat owners in Japan.
I hope that they could feel secure in their lives with their loved cats.
https://tolettacat.com/blogs/toletta-blog/with-covid19-20200615

Current situation and Future of Veterinary Medicines and Related Industries in Japan and the US – New Technologies that Change Future –
The captioned article has been written by Dr. Yuki Ujimasa, DVM, MS, President of AHRMS, Inc., and published on the Journal of the Japan Veterinary Medical Association since October 2019. It recently became public via the following link:
http://nichiju.lin.gr.jp/mag/07210/a4.pdf
Sorry to say, it is described only in Japanese. The chapters of the article are as follows:
1. Recent activities by multi-national animal health pharmaceutical companies and domestic companies
2. New technologies that can change husbandry industries
3. Impact of emerging and re-emerging infectious diseases in food animals and Possibilities of plant-based meat and cultured meat
4. Telemedicine in veterinary medicines
5. Changes of distribution system and home-delivery in companion animal medicines
6. Necessity for review of veterinary advertising regulations
7. Conclusions
8. Acknowledgment
Seminar for Japan Pharmacist Association
Dr. Yuki UJIMASA, DVM, MS of AHRMS, Inc. gave presentations titled “Japan in the World, Trends in Animal Medicines and Veterinary Science” at seminar for Japan Pharmacist Association on February 7th in Tokyo and 14th in Osaka.

Animal Health Innovation Asia 2019
We had the 4th Animal Health Innovation Asia 2019 in Tokyo on November 6th and 7th by receiving more than 150 attendee.
We were very happy to support the event as Consulting Partner.
Dr. Yuki Ujimasa, DVM, MS chaired a panel discussion and give two presentations as President of AHRMS, Inc. and President of Vm3, Inc., respectively.

Animal Health Innovation Asia 2019

Animal Health Innovation Asia 2019
Animal Health Innovation Asia 2019 will be held on November 6th and 7th at Hotel Westin Tokyo.
We, AHRMS, Inc., are supporting the event as “Consulting Partner” to fix the program, introduce Japanese start-ups and new technologies to multi-national animal health manufacturers and facilitate those companies to enter Japanese animal health markets.
- In progress:
■Applications of oral presentations by Japanese TLOs and Start-ups (until June 25th)
■Recruiting of Sponsor companies - Program
■Dr. Ken Noda of NVAL, JMAFF
■Dr. Masato Sakai of JVMA
■Dr. Hiroshi Oishi of JVPA - Website:
https://www.animalhealthasia.com/events/animal-health-investment
(Kisaco Research)

Zoetis leads Japanese approvals in 2018 and across 10-year scope
News by Mr. Joseph Harvey, Editor of Animal Pharm
Zoetis received three approvals from the Japanese Ministry of Agriculture, Forestry and Fisheries (JMAFF) in 2018, bolstering its position as the firm with the most new products in Japan over the last 10 years.
The JMAFF approved 14 new animal drugs in 2018. These products included five antimicrobials, four pharmaceuticals, three biologicals and two antiparasitics. There were eight approvals for livestock and six authorizations for companion animals.
The number of JMAFF approvals is down by one in comparison to 2017’s total.
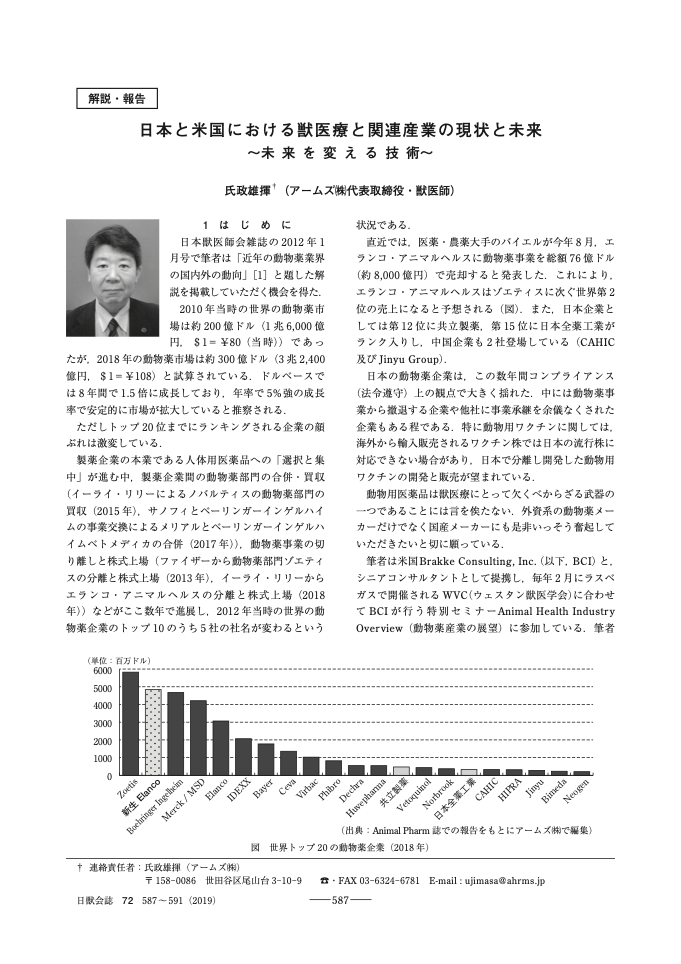
Regarding marketing approvals for new animal health products in Japan between 2009 and 2018, Tokyo-based animal health consultancy AHRMS said Zoetis had received the most.
“Zoetis leads Japanese approvals in 2018 and across 10-year scope” の続きを読む
